
The US FDA New Drug Approvals in December 2024
Shots:
-
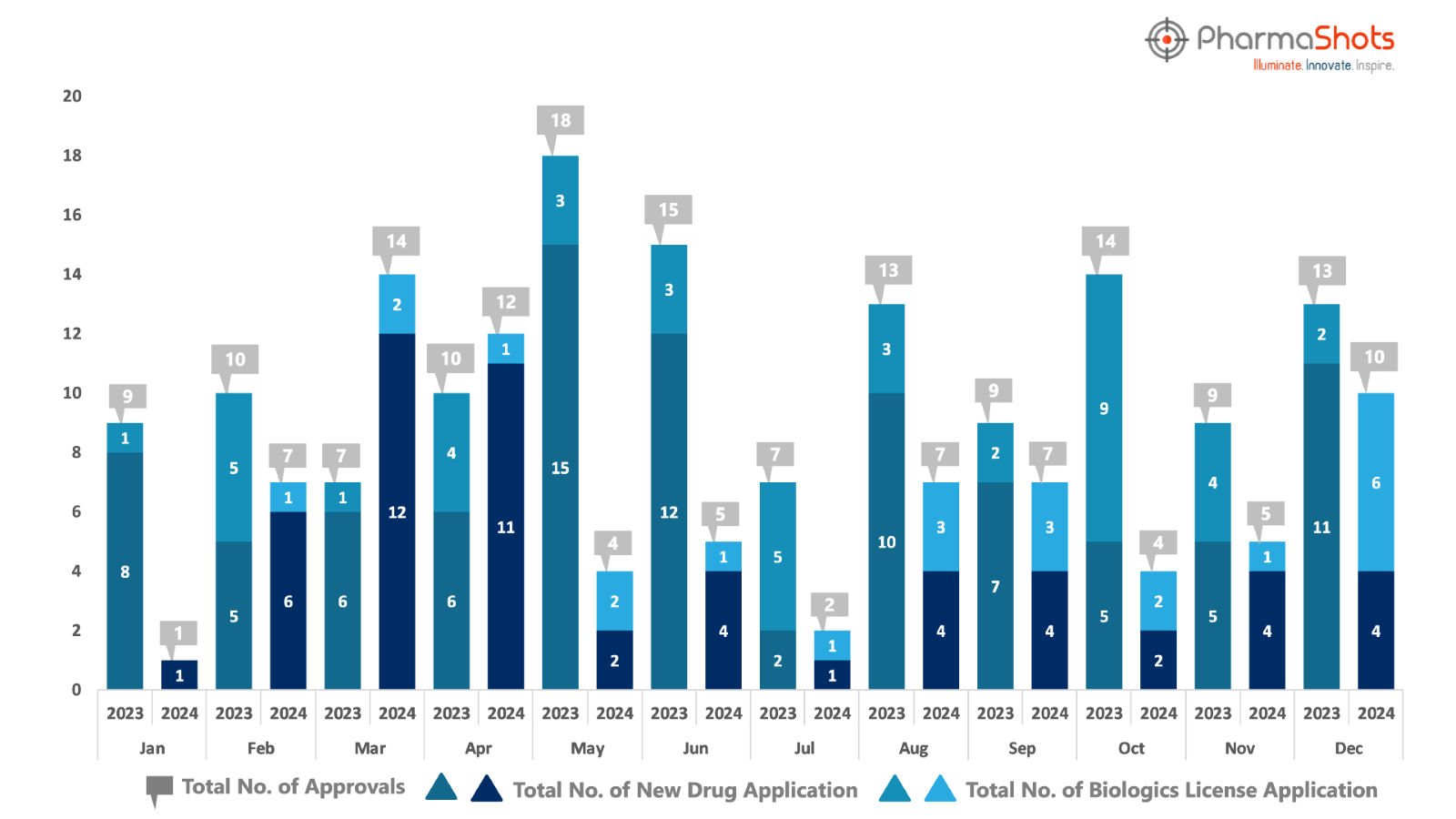
PharmaShots has compiled a list of US FDA-approved drugs in the month of December 2024
-
The US FDA has approved a total of 10 new drugs including 4 new molecular entities and 6 biologics leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drug was BMS’ Opdivo Qvantig securing approval for treating G/GEJ Cancers
Product Name: Bizengri
Active ingredient: Zenocutuzumab-zbco
Company: Merus
Disease: Pancreatic Adenocarcinoma and NSCLC
Date: Dec 04, 2024
Shots:
-
The US FDA has granted accelerated approval to Bizengri for the treatment of advanced, unresectable or metastatic NRG1+ pancreatic adenocarcinoma or NSCLC. It will be commercialized by Partner Therapeutics in the US under previously signed agreement
-
Approval was based on eNRGy study assessing the safety, tolerability, PK/PD, immunogenicity & anti-tumor activity of zenocutuzumab to treat NRG1+ solid tumors incl. pancreatic cancer (n=30) & NSCLC (n=64) in patients, progressed on prior treatment
-
Study demonstrated an ORR of 40% & DoR range from 3.7 to 16.6mos. in pancreatic cancer patients while ORR of 33% & mDoR of 7.4mos. in NSCLC patients
Product Name: Crenessity
Active ingredient: Crinecerfont
Company: Neurocrine Biosciences
Disease: Congenital Adrenal Hyperplasia
Date: Dec 13, 2024
Shots:
-
The US FDA’s approval of Crenessity as an adjunctive treatment to glucocorticoid replacement to treat CAH was based on a global P-III (CAHtalyst Pediatric: n=103, age: 4-17yrs. & Adult: n=182, age: 18-58yrs.) trials. It has also received PRV on approval
-
Pediatric study achieved its 1 & 2EP, depicting ~4x greater reductions in androstenedione at wk.4 & GC doses (with improved androgen levels) at wk.28 as well as ~12x greater reduction in 17-OHP vs PBO
-
Adult study also met its 1 & 2EP, showing 63% vs 18% (PBO) achieving physiologic GC doses with ~2x greater steroid dose reduction at wk.24, ~8x greater reduction in androstenedione levels at wk.4 and ~37x greater reduction in 17-OHP vs PBO
Product Name: Unloxcyt
Active ingredient: Cosibelimab-ipdl
Company: Checkpoint Therapeutics
Disease: Metastatic or Locally Advanced Cutaneous Squamous Cell Carcinoma
Date: Dec 13, 2024
Shots:
-
Checkpoint has received the US FDA’s approval for Unloxcyt (1,200mg, IV, over 60 minutes, Q3W) to treat metastatic or locally advanced cSCC in adults, ineligible for curative surgery or radiation
-
Approval was based on the P-I (CK-301-101) trial in solid tumor patients (incl. cSCC: N=109), showing ORR of 48% with mDoR of 17.7mos. in locally advanced cSCC patients (n=31) & ORR of 47% with mDoR not achieved in metastatic disease patients (n=78)
-
Unloxcyt is a human, anti-PD-L1 IgG1 mAb which blocks the interaction between PD-L1 & T cell receptors (PD-1 & B7.1) producing an anti-tumor immune response
Product Name: Nemluvio
Active ingredient: Nemolizumab
Company: Galderma
Disease: Moderate-to-Severe Atopic Dermatitis
Date: Dec 13, 2024
Shots:
-
The US FDA has approved Nemluvio + TCS ± calcineurin inhibitors (TCI) to treat mod. to sev. atopic dermatitis in patients unresponsive to topical therapies
-
Approval was based on the P-III (ARCADIA) study of the combination vs PBO in patients (n=1,728; ≥12yrs.), showing significant skin clearance in both co-1EPs of IGA score (0/1) & 75% EASI reduction at wk.16.; 2EPs of itch relief & improved sleep disturbance, were also met
-
Nemluvio has received the CHMP's positive opinion in Dec 2024 for AD & prurigo nodularis. Submissions in Australia, Singapore, Switzerland, Canada, Brazil & South Korea are under review, with more expected in 2025
Product Name: Ensacove
Active ingredient: Ensartinib
Company: Xcovery Holdings
Disease: ALK-Positive, Locally Advanced or Metastatic NSCLC
Date: Dec 18, 2024
Shots:
-
Xcovery Holdings has received the US FDA’s approval for Ensacove (225mg, oral, QD) for ALK+, locally advanced or metastatic NSCLC in patients not previously treated with ALK-targeted therapy
-
Approval was based on a global P-III (eXalt3) study assessing ensartinib vs crizotinib in 290 patients, demonstrating significantly improved PFS with mPFS of 25.8 vs 12.7mos. (1EP) & similar OS for both arms (2EP)
-
Ensartinib is an ALK tyrosine kinase inhibitor (TKI) developed by Xcovery & Betta Pharmaceuticals
Product Name: Tryngolza
Active ingredient: Olezarsen
Company: Ionis Pharmaceuticals
Disease: Familial Chylomicronemia Syndrome
Date: Dec 19, 2024
Shots:
-
The US FDA has approved Tryngolza (80mg, QM, self-administered via an auto-injector) as an adj. for reducing triglycerides (TG) in FCS patients, based on a P-III (BALANCE) study. Further submission is under review in the EU, with more filings planned
-
The P-III study involved FCS patients with fasting TG levels of ≥880mg/dL. Trial showed PBO-adjusted mean reduction in TG levels of 42.5% (6mos.) & 57% (12mos.) plus reduced AP events at 12mos. with 5% (1 pt) on Tryngolza having 1 episode vs 30% (7 pts) on PBO having 11 AP episodes
-
Tryngolza (RNA-targeted drug) reduces apoC-III production responsible for regulating TG & is being evaluated in 3 P-III trials (CORE, CORE2 & ESSENCE) to treat sHTG
7. The US FDA Approves Vertex’s Alyftrek for the Treatment of Cystic Fibrosis
Product Name: Alyftrek
Active ingredient: Vanzacaftor/Tezacaftor/Deutivacaftor
Company: Vertex Pharmaceuticals
Disease: Cystic Fibrosis
Date: Dec 20, 2024
Shots:
-
The US FDA has approved Alyftrek (QD, CFTR modulator) to treat patients (≥6yrs.) with cystic fibrosis (CF), having at least one F508del mutation or another CFTR gene mutation. Submissions are under review in the EU, UK, Canada, Switzerland, Australia & New Zealand
-
Approval was supported by pivotal P-III CF program involving >1,000 patients across 20+ countries, with results reported as conclusion of the studies & highlighted at the North American Cystic Fibrosis Conference in Sep 2024
-
P-III studies in CF patients (≥12yrs.) showed that Alyftrek met its 1EP of ppFEV1 non-inferiority & 2EP of SwCl improvement vs Trikafta. In children (6–11yrs.), the drug demonstrated safety (1EP) as well as benefits in ppFEV1 & SwCl
Product Name: Alhemo
Active ingredient: Concizumab-mtci
Company: Novo Nordisk
Disease: Hemophilia A or B
Date: Dec 20, 2024
Shots:
-
The US FDA has approved Alhemo injection (QD) as a prophylactic treatment to reduce the frequency of bleeding episodes in hemophilia A or B patients (≥12yrs.) with inhibitors
-
Approval was based on the P-III (explorer7) study assessing Alhemo's efficacy and safety by comparing annual bleeding rates (ABR) in patients aged 12+ with hemophilia A or B with inhibitors
-
Study showed Alhemo reduced annual bleeding rates (ABR) by 86%, with mean ABR of 1.7 vs 11.8. The overall median ABR was 0 vs 9.8 without prophylaxis, and 64% of patients on Alhemo had no spontaneous and traumatic bleeds in the first 24wks., vs 11% without prophylaxis
Product Name: Tevimbra + CT
Active ingredient: Tislelizumab-jsgr
Company: BeiGene
Disease: G/GEJ Cancers
Date: Dec 26, 2024
Shots:
-
The US FDA has approved Tevimbra + Pt & fluoropyrimidine-based CT as a 1L treatment of inoperable or metastatic HER2- G/GEJ adenocarcinoma with PD-L1 (≥1) expression. Another BLA for 1L locally advanced unresectable or metastatic ESCC is under review
-
Approval was based on P-III (RATIONALE-305) study of Tevimbra + CT vs PBO in patients (n=997) that achieved 1EP, showing OS benefits with a 20% reduction in the death risk & mOS of 15 vs 12.9mos.
-
Pooled safety analysis included data from 2 studies (RATIONALE-302 & BGB-A317-303) in 1,972 patients plus 5 trials (BGB-A317-208, BGB-A317-204, BGB-A317-203, BGB-A317-102 & BGB A317_Study_001) in patients with ESCC (n=307) & advanced or recurrent tumors (n=1,665)
10. BMS’ Opdivo Qvantig SC Injection Secures the US FDA’s Approval for Solid Tumor Opdivo Indications
Product Name: Opdivo Qvantig
Active ingredient: Nivolumab; Hyaluronidase-nvhy
Company: BMS
Disease: G/GEJ Cancers
Date: Dec 27, 2024
Shots:
-
The US FDA has approved Opdivo Qvantig (nivolumab & hyaluronidase-nvhy) SC injection for previously approved solid tumor Opdivo indications, incl. as monotx., maintenance therapy after Opdivo-Yervoy combination & with CT or cabozantinib
-
Approval was based on P-III (CheckMate-67T) study assessing Opdivo Qvantig (nivolumab: 1,200mg & hyaluronidase: 20,000 units, SC, Q4W; n=248) vs Opdivo (3mg/kg, IV, Q2W; n=247) in adults (N=495) with advanced or metastatic ccRCC treated previously with systemic therapy
-
Study depicted noninferiority in co-1EPs of Cavgd28 (GMR: 2.10) and Cminss (GMR: 1.77). The ORR was 24% vs 18%, showing comparable efficacy
Related Post: Insights+: The US FDA New Drug Approvals in November 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














